Peptides: The Broscience Guide to Retatrutide
Disclaimer
The information contained in this article is provided for educational and entertainment purposes only. It is not intended as medical advice, diagnosis, or treatment. The content represents a synthesis of publicly available research, anecdotal reports, and biohacker perspectives, and is not a substitute for professional medical guidance.
Retatrutide is an investigational compound not currently approved for general medical use outside of clinical trials. Off-label or unsupervised use may involve significant risks.
Always consult a qualified healthcare professional before starting any new health protocol, medication, or supplementation.
Section 1: Introduction
The New Era of Fat Loss and Metabolic Control
Since the introduction of GLP-1s to the general public, the science and practice of fat loss has changed. Semaglutide and then Tirzpetatide have enabled millions of people to successfully lose bodyfat. These compounds have been controversial, but used correctly as part of a holistic approach, their effectiveness is undeniable. The next peptide that will be hitting the market will be Retatrutide, colloquially known as “Reta”. This is the 8th generation fat loss peptide that has become massively popular in the biohacking and bodybuilding world the past year. While currently only available for scientific research purposes as a research compound, its being used off-label for fat loss, and its effects surpass those of Semaglutide and Tirzepatide.
With its triple-receptor action, it outperforms the prior GLP1s (I am using this term generically), targeting not just appetite and insulin, but also fat oxidation and energy expenditure.
Currently in Phase 3 clinical trials, Retatrutide has already demonstrated unprecedented weight loss (up to 24%), reversal of fatty liver disease, and biomarker improvements that suggest possible cardiovascular protection.
While not FDA-approved as of 2025, it has already become a favorite tool among elite biohackers for rapid body recomposition, appetite control, and metabolic health restoration.
This guide is a simple overview of the current research, and protocols on Reta, both clinical and anecdotal.
Section 2: What Is Retatrutide?
Lets start with the basics. What is it?
Reta is a synthetic peptide developed by Eli Lilly. It's part of the class of drugs called incretin-based therapies, so named because they mimic the gut-derived hormones (incretins) that regulate blood sugar and appetite.
Retatrutide is a single, synthetic peptide molecule. It mimics the signaling of multiple endogenous hormones. It includes a fatty acid side chain (a diacid linker) that binds to albumin in the blood, prolonging its action and allowing for once-weekly dosing.
It simultaneously activates three different receptors:
GLP-1 receptor
GIP receptor
Glucagon receptor
This triple-agonist architecture is what sets it apart from earlier agents like:
Semaglutide (GLP-1 only)
Tirzepatide (GLP-1 + GIP)
This is accomplished through cutting edge amino acid sequence design and structural modifications that allow the peptide to bind to and activate all three receptors with high affinity, while also maintaining stability and a long half-life in the bloodstream. A 3 in 1 peptide essentially.
This triple synergistic effect goes beyond what the previous drugs could achieve.
Reta was designed to treat obesity, type 2 diabetes (T2D), and fatty liver disease, specifically non-alcoholic fatty liver disease (NAFLD) and its more advanced form, metabolic dysfunction-associated steatohepatitis (MASH).
Like the other GLP1s, the motivation with Reta is reversing obesity and metabolic diseases by addressing insulin resistance, chronic inflammation, and excess fat.
I say this to make a point: these compounds exist to help people become HEALTHIER. That is their entire reason for existence.
Traditional treatments and approaches have been slow-acting, incomplete, or hard to sustain. Reta and the other GLP1s are often maligned as “short cuts”, but the ideal treatment approach for these compounds is holistic and used over months to get someone into a leaner, healthier, sustainable state.
How Retatrutide Works
Triple receptor agonism is the engine behind Retatrutide’s effects. Each receptor it targets contributes something vital to the process of weight loss, energy balance, and metabolic repair.
1. GLP-1 Receptor Activation
This is the same target as semaglutide (Ozempic, Wegovy). GLP-1, or glucagon-like peptide-1, is a hormone your gut releases when you eat. It tells your pancreas to release insulin, slows down how quickly your stomach empties, and sends appetite-suppressing signals to your brain.
By stimulating the GLP-1 receptor, Retatrutide helps:
Increase insulin secretion (but only when blood sugar is elevated, this reduces risk of hypoglycemia)
Suppress glucagon, a hormone that raises blood sugar
Slow gastric emptying, leading to longer satiety
Dampen appetite through central nervous system pathways
This alone is enough to drive meaningful weight loss and blood sugar control. Its how Semaglutide works after all. But Retatrutide does not stop here.
2. GIP Receptor Activation
GIP stands for glucose-dependent insulinotropic polypeptide. It’s another gut hormone that boosts insulin release and has its own effects on fat metabolism. GIP is one of the two primary “incretin” hormones released from the gut in response to food intake (the other being GLP-1). It’s secreted by K-cells in the proximal small intestine shortly after nutrients, particularly fats and carbohydrates, enter the digestive tract.
GIP binds to the GIP receptor (GIPR), a Gs-coupled G-protein-coupled receptor (GPCR) expressed on pancreatic β-cells, adipocytes, bone cells, and parts of the central nervous system. Upon binding, it turns on adenylyl cyclase, increasing intracellular cAMP, and initiating signaling cascades that influence insulin secretion, lipid metabolism, and energy homeostasis. Sounds complicated? It is, but the downstream effects are what we care about
Enhancing insulin secretion, especially after meals (improves insulin sensitivity)
Improving lipid handling, making it easier for the body to burn fat (improves fat oxidation)
Supporting lean mass retention, helping to prevent muscle loss during weight loss
Reducing tachyphylaxis, aka the diminishing response effect. This is the issue with semaglutide, which plateaus and causes side effects.
Retas GIP activity works to buffer and amplify the benefits of GLP-1, preventing tolerance and making results more sustainable. This is why Tirzepatide works so well, but Reta one ups it.
3. Glucagon Receptor Activation
This is what makes Reta unique. Glucagon is a hormone produced by the pancreas that plays a critical role in regulating blood sugar and energy metabolism.
It acts as a counterbalance to insulin, raising blood glucose levels when they drop too low by triggering the breakdown of glycogen in the liver (glycogenolysis) and stimulating the creation of new glucose from non-carbohydrate sources (gluconeogenesis).
It also mobilizes stored energy by promoting fat breakdown (lipolysis) and ketone production, especially during fasting or low-carb intake. It helps the body shift from burning carbohydrates to burning fat, enhancing metabolic flexibility.
What does that add up? By prompting gluconeogenesis and increased fat oxidation, reta has a unique effect of keeping metabolism ELEVATED when normally it would be dropping while dieting.
Raises resting energy expenditure, helping the body burn more calories
Promotes lipolysis, the breakdown of stored fat
Activates brown adipose tissue, increasing thermogenesis
Reduces liver fat, a key goal in NAFLD and MASH treatment
Hence the Triple Threat Designation
By combining all three of these hormonal signals into a single molecule, Retatrutide is impacting metabolism across multiple markers and systems. In clinical trials, this has translated to:
Significant reductions in body weight, even in individuals with severe obesity
Improved blood sugar and insulin sensitivity, especially in people with type 2 diabetes
Marked reductions in liver fat
Preservation of muscle mass, which is often lost with calorie restriction or rapid weight loss
What sets Retatrutide apart isn’t just how much weight people lose, it’s how they lose it. Appetite is reduced, no question, but the body also burns more energy, processes carbs and fats more efficiently, and reverses dangerous fat deposits around organs like the liver and pancreas.
How It’s Used
Retatrutide is administered as a once-weekly subcutaneous injection. Thanks to a fatty diacid side chain, the molecule binds to albumin in the blood, extending its half-life and keeping levels stable across the week. This means fewer peaks and troughs, less nausea, and a more predictable clinical effect.
While it’s still investigational as of mid-2025, ongoing Phase 3 trials suggest Retatrutide is going to become the new gold standard in obesity and metabolic disease treatment.
Section 3: Clinical Research and Results
Anytime people talk about peptides online, there is hype and you're listening to someone’s N=1 results. The general public rarely knows how much clinical research has gone into GLP-1s. Contrary to misconception, they have been used for over 20 years in medicine, and are very safe prescriptions.
But what about Reta specifically? How do we KNOW it works? We have DATA.
A Chronological Overview of Retas Human Trials
Phase 1
The Phase 1 trial data for Reta was released back in 2022. A Phase trial is to assess basic safety for any new drug, and how it behaves in the actual body.
The Phase 1 trial enrolled 72 adults between the ages of 20 and 70 who had type 2 diabetes and were either overweight or obese. All participants had relatively stable body weight for at least three months prior. Over the 12-week period, they received one of the following:
-a placebo (an inactive shot)
-dulaglutide (an approved diabetes drug for comparison)
-or one of several escalating doses of retatrutide—ranging from low (0.5 mg) to high (up to 12 mg).
The goal was to see how people tolerated the drug, how it affected their blood sugar and weight, and how it was processed by the body.
The results were encouraging.
People in the higher dose groups saw a significant drop in blood sugar levels, around 2.8 to 3.1 mmol/L lower on average, compared to those on placebo.
Long-term blood sugar control, measured by HbA1c (a marker of average glucose over 2–3 months), also improved meaningfully. In the three highest dose groups, HbA1c dropped by between 1.2% and 1.6% in 12 weeks, which is comparable to or better than many existing diabetes medications.
These improvements happened without causing dangerous low blood sugar levels (hypoglycemia), which is a major concern with some diabetes treatments.
Participants also lost weight, and the effect was clearly dose-dependent—the higher the dose, the more weight people lost.
In the group receiving the most retatrutide (gradually escalating to 12 mg), average weight loss was nearly 9 kilograms (about 20 pounds) by week 12. That’s an impressive result for such a short trial and suggests that the drug has powerful effects on fat metabolism and appetite.
To note, this was also without any exercise or diet guidance.
As with other GLP1s, gastrointestinal side effects were common.
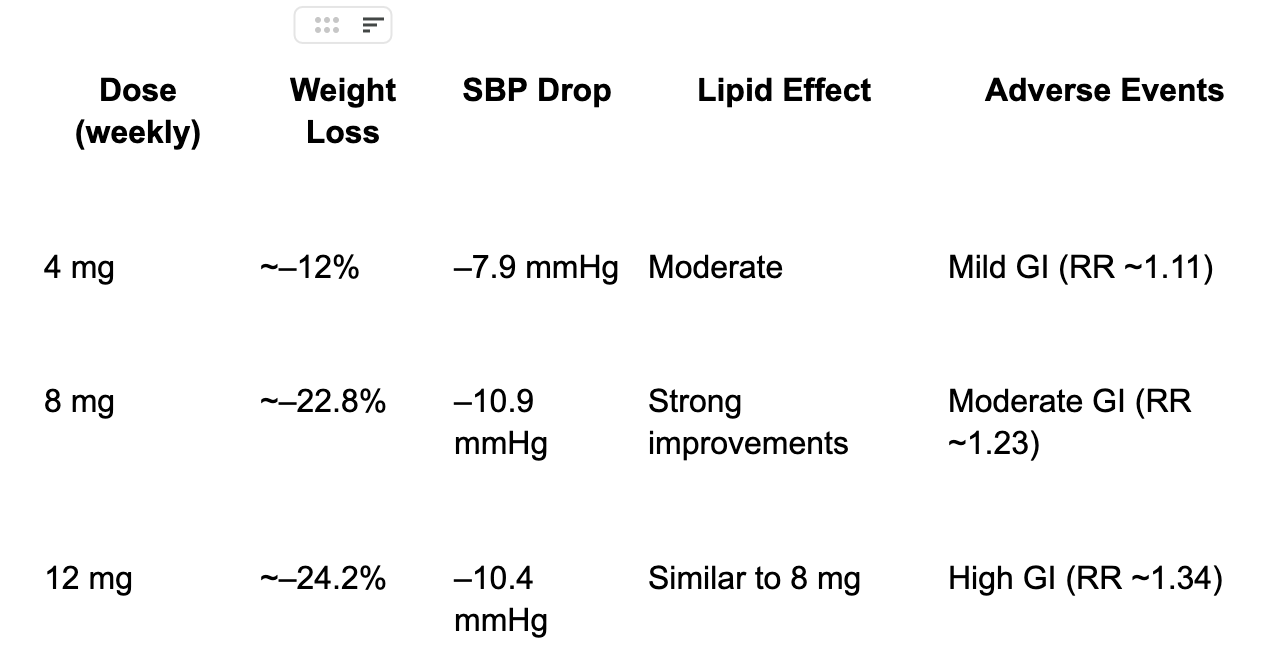
About 63% of people taking retatrutide experienced some form of GI discomfort, this included nausea, vomiting, and diarrhea. These side effects were typically experienced in the first month and when the dose was being increased, and tended to subside after.
For context, about 60% of those on dulaglutide and 54% on placebo reported similar symptoms. Most side effects were not severe and tended to improve over time.
In terms of how the drug behaves in the body, reta has a demonstrable half-life of about 6 days, meaning it stays active in the bloodstream long enough to only require a once-weekly injection, a convenient schedule for patients.
Overall, this early trial showed that retatrutide is generally safe, well-tolerated, and was capable of improving both blood sugar and weight in people with type 2 diabetes.
The results were strong enough to justify multiple Phase 2 studies, and how future trials would be designed. It also confirmed that triple-agonist drugs could be a major step forward in the treatment of obesity and metabolic disease.
Phase 2 Obesity Trial (NCT04881785)
The Phase 2 trial data was published in The New England Journal of Medicine, 2023
This study was larger and included 338 adults with a BMI ≥30, or ≥27 with at least one obesity-related condition such as hypertension or insulin resistance.
This study was to look at the weight loss effects specifically.
Participants were randomly assigned weekly subcutaneous injections of Retatrutide across a dose range of 1 to 12 mg, over a 48-week period.
48 weeks is 11 months. This study was testing not just the short term weight loss effects, but how long Reta could effectively be used.
Key Outcomes:
The Phase 2 study was what made major waves in the medical and biohacker community.
At 12 mg, average body weight loss reached 24.2%, translating to an average of ~58 lbs lost.
50% of participants at the highest dose lost ≥25% of body weight—an outcome rarely seen outside of bariatric surgery.
HbA1c dropped by just over 2 percentage points, with dose-dependent improvement across the range.
Liver fat resolution occurred in 86% of participants at 12 mg.
Blood pressure, triglycerides, and liver enzymes all improved significantly.
The Dose–Response Optimization
This trial was critical for revealing a clear dose–response relationship with side effects. The weight loss at the highest dose was only 2% higher at a ⅓ lower dose, with less side effects.
The 4-8 mg dose emerged as the therapeutic “sweet spot”, offering near-maximal efficacy with significantly fewer gastrointestinal side effects than 12 mg.
Phase 2 Non Alcoholic Fatty liver Disease/MASH Trial
This was a companion study focused on participants with non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated steatohepatitis (MASH). Dosed similarly to the obesity trial, Retatrutide produced one of the most robust liver responses observed in a metabolic therapeutic.
Key Outcomes:
Liver fat reduction: 81–86% across higher dose groups.
93% of participants achieved a ≥30% reduction in hepatic fat, the threshold typically associated with histological improvement.
Fibrosis-related biomarkers also improved, suggesting not only fat clearance but also reduced scarring—a rare outcome in liver trials.
This trial pointed to Reta being the most efficacious treatment in medicine for NAFL and MASH. This led to the Phase 3 trials
Phase 3 TRIUMPH Clinical Program (Ongoing)
Following these successes, Eli Lilly launched the TRIUMPH Phase 3 series to evaluate Retatrutide in broader, more diverse populations:
TRIUMPH-1 through TRIUMPH-4:
Investigate weight loss and metabolic health in participants with comorbidities like T2D, obstructive sleep apnea, and cardiovascular disease. Primary endpoints include % weight change, metabolic markers, and quality of life measures.TRIUMPH-Outcomes (NCT06383390):
A long-term cardiovascular outcomes trial enrolling ~10,000 patients with established ASCVD or chronic kidney disease.Primary endpoints: Time to first major adverse cardiovascular event (MACE: CV death, MI, stroke) and composite renal failure outcomes.
Estimated completion: Early 2029
Section 4: Side Effects, Contextualized
The question of side effects with GL-1s gets brought up A LOT online. While its true that side effects happen, there is a massive degree of sensationalism and fabrication. GLP1s are not making hundreds of people going blind, causing an epidemic of cancer, or leading to thousands of lawsuits.
First, it must be said that with all incretin-based therapies, gastrointestinal symptoms were the most commonly reported adverse events.
These symptoms are not mysterious and are well known in the medical literature
Second, speaking professionally, I have had dozens of clients use these GLP1s, and received feedback from hundreds of biohackers. Ive consulted with doctors who prescribe GLP1s regularly.
What has emerged is an understanding that while side effects do happen, they are often attributable to poor habit and lifestyle practices, or excessive dosing
So what Side effects are we talking about here?
Common adverse effects:
Nausea
Vomiting
Diarrhea or constipation (transient)
Slight heart rate increase
Mild insomnia
Why is this happening?
These side effects may sound scary initially, but the biohacker community figured out quickly that almost all of them were avoidable
1. The primary reason these side effects emerged in clinical trials was from TOO high dosing.
Similar to Semaglutide and Tirzepatide, taking too much, too soon causes side effects.
The best practice with Reta is to start with 1mg, once a week dosing, and ONLY increase as needed. Follow the protocols as listed in Chapter 6.
2. The next reason that caused side effects to emerge was lack of hydration
Reta is a powerful appetite suppressant, not just for food, but also fluids. When people eat less food, they also consume less water as a result. Both from beverages and from water content in food.
Solution?
Drink half your bodyweight in water daily, and consume sufficient electrolytes, at least 2-5 grams of sodium and 3-5 grams of potassium (the more active you are, the more sodium you will need).
I suggest LMNT packets mixed into 1 liter of water. Salt your food liberally.
Do not wait to feel thirsty.
Make sure to consume adequate magnesium. I suggest magnesium glycinate, anywhere from 200-400mg+ a day.
The elevated heart rate, nausea, and indigestion are mitigated immensely by this.
If HR increases, that is NORMAL
Reta crosses the blood brain barrier, and stimulates the central nervous system. This often leads to an increase in resting heart rate, usually around 5 beats per minute. This is normal, and a sign that the Reta is working, and that metabolism is elevated. This effect typically fades by the end of the first month.
This HR increase can be mitigated by lowering intake of caffeine and other stimulants, and following the prior recommendations for fluid intake and electrolytes, especially magnesium.
3. People don't consume enough fiber, and eat overly fatty foods
Reta is similar to Semaglutide and Tirzepatide in that it particularly slows down digestion of fatty foods.
The combination of appetite suppression plus poor dietary planning leads to indigestion, and diarrhea.
Solution? Increase soluble fiber intake. Eat a low fat to moderate fat diet (20-30% of total calories).
Top 20 High-Fiber Foods (Soluble + Insoluble)
Oats
Barley
Psyllium husk
Chia seeds
Flaxseeds
Black beans
Lentils
Kidney beans
Brussels sprouts
Sweet potatoes (with skin)
Apples (with skin)
Pears (with skin)
Avocados
Carrots
Whole wheat bread
Brown rice
Popcorn (air-popped)
Broccoli
Almonds
Berries (especially raspberries & blackberries)
4. Reta should be administered in the MORNING
Because Reta is metabolically stimulating, it is best to use in the morning. Taking it in the PM can lead to disturbed sleep.
Insomnia is rarely an issue with Reta. Rather, people find they sleep a bit less. To ensure deep sleep, consume adequate magnesium, glycine, and theanine before bed.
I recommend PreSleep for this.
Like the elevated HR, this effect on sleep fades by the end of the first month typically.
5. If nausea does noticeably happen in the first weeks, try consuming crystallized ginger
Ginger is a natural anti nausea solution.
6. If side effects make you uncomfortable and hesitant, it is OKAY to not research Reta
As any professional will tell you in medicine or health, do not do something if you are not comfortable with it.